Epithelial unjamming triggered by mechanical stress
|
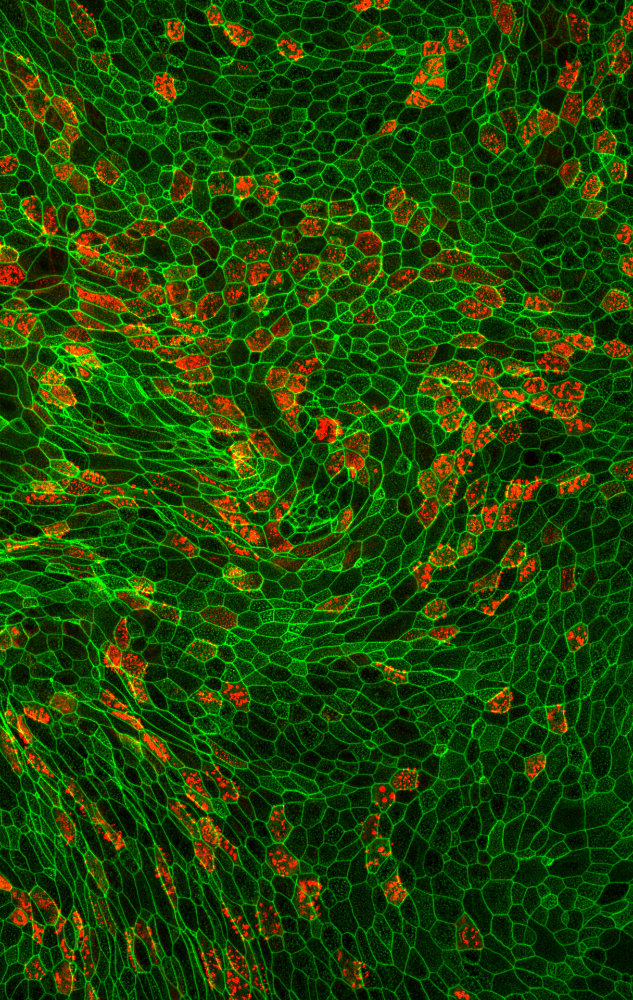
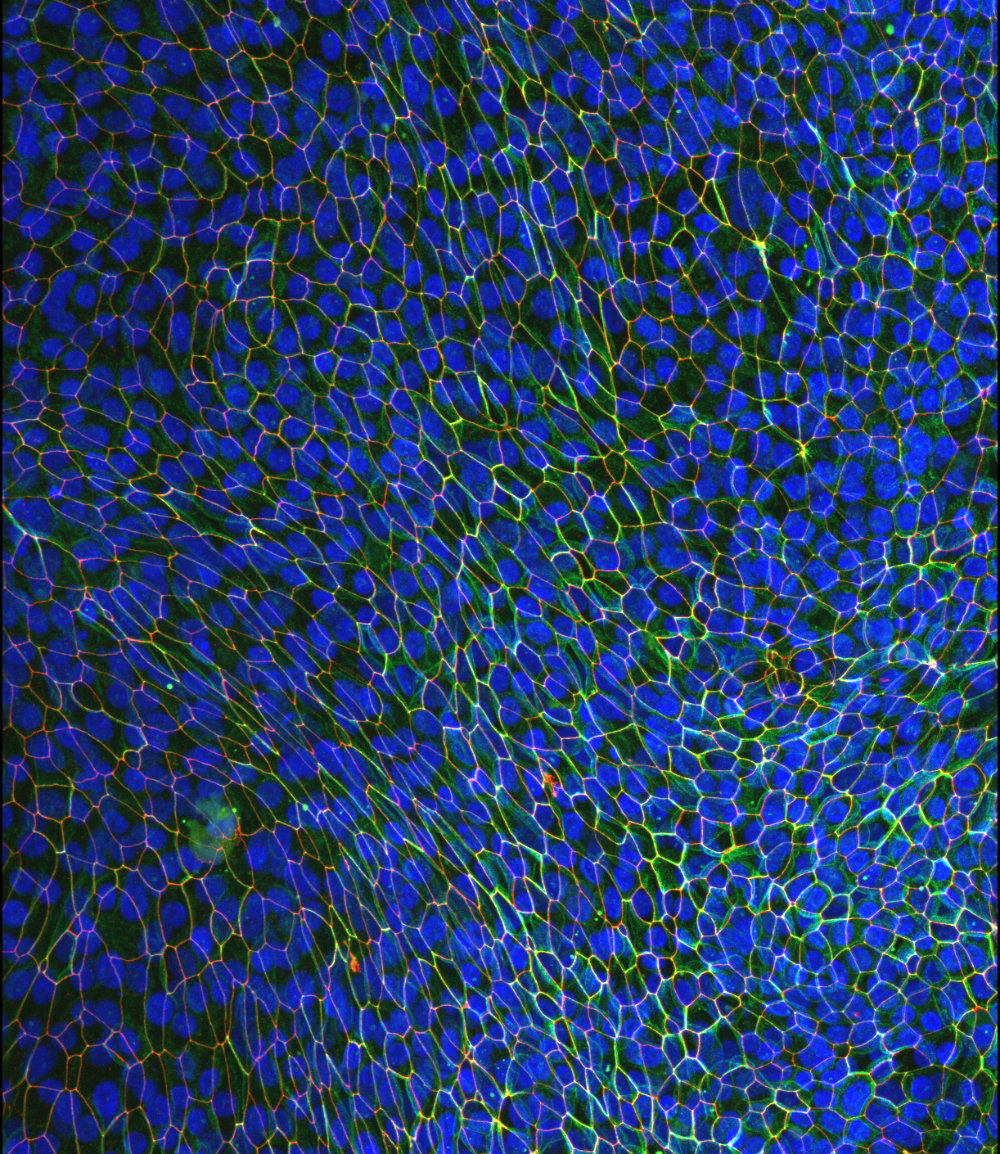
I joined the labs of Drs. Park and Fredberg after learning of their recent discovery of cellular jamming, a physical phenomenon in which an epithelial cell layer can exist in a phase that is solid-like, non-migratory, and jammed, or adopt a phase that is fluid-like, migratory, and unjammed. Inspired by an a-priori prediction from an in-silico model of cell jamming (Bi, et al, 2014), I discovered a systematic relationship between cell jamming and cell shape: stationary jammed layers were comprised of isotropic, roughly hexagonal cells, whereas migratory unjammed layers were comprised of elongated, variably shaped cells (Park, et al, 2015).
I next focused on the important unanswered question: what is the relationship between the newly discovered unjamming transition (UJT), with the well-studied epithelial-to-mesenchymal transition (EMT)? Both EMT and UJT enable epithelial migration, plasticity, and remodeling, but the extent to which these programs are distinct, overlapping, or even identical, was unknown. I demonstrated that, through the UJT, a differentiated epithelial cell is able to swap positions with neighbors and migrate collectively, without undergoing EMT (Mitchel et al, Nature Comms, 2020). I showed that differentiated primary epithelial cells, whose primary function is assumed to be maintenance of a tight barrier, are able to migrate as a collective, even while maintaining adhesive connections to their neighbors. Despite residing in a crowded tissue, where there is no free space to move, epithelial cells can be triggered to unjam through changes in cell shape and local self-organization. |
Mechanotransduction in asthma pathogenesis: a vicious cycle
|
In response to mechanical compression that occurs during bronchoconstriction, airway epithelial cells begin to effect processes that ultimately result in the structural changes that are associated with asthmatic airways, including goblet cell hyperplasia and basement membrane thickening.
I have shown that when the airway epithelium is compressed, these cells secrete pro-asthma mediators that can, in turn, cause a pathological response in neighboring cells (Mitchel, et al, 2016). In particular, compressed epithelial cells secrete factors which cause airway smooth muscle hyperplasia and hyperreactivity (Lan*, Mitchel*, et al, 2018). This in turn may increase the likelihood or magnitude of future asthma attacks, leading to additional mechanical compression. We hypothesize that asthmatic disease progression may be a vicious positive feedback loop where airway epithelium and smooth muscle communicate through biological and physical mechanisms to initiate and propagate disease pathophysiology. |